Answer:
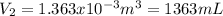
Step-by-step explanation:
Hello,
In this case, since the work done at constant pressure as in isobaric process is computed by:
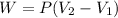
Thus, given the pressure, initial volume and work, the final volume is:
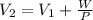
Whereas the pressure must be expressed in Pa as the work is given in J (Pa*m³):
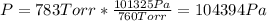
And the volumes in m³:
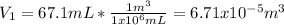
Thus, the final volume turns out:
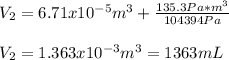
Best regards.