Answer:
The molecular weight of the gas mixture is 35.38 g/mol.
Step-by-step explanation:
The molecular weight of the gas can be found using the following equation:
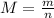
Where:
m: is the mass = 230 g
n: is the number of moles
First, we need to find the number of moles using Ideal Gas Law:
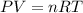
Where:
P: is the pressure = 135 psi
V: is the volume = 15 L
R: is the gas constant = 0.082 L*atm/(K*mol)
T: is the temperature = 465 °R (K = R*5/9)
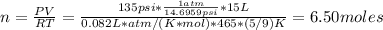
Finally, the molecular weight of the gas is:
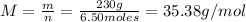
Therefore, the molecular weight of the gas mixture is 35.38 g/mol.
I hope it helps you!