Answer:
1.375%
Step-by-step explanation:
Percent Error = measured value - accepted value/ accepted value x 100
Measured Value: 15.78
Accepted Value: 16.00
Work:
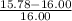 * 100
* 100
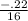 * 100 = -1.375
* 100 = -1.375
You cannot have a negative percentage. Therefore the answer is 1.375%