Given :
Normal atom.
To Find :
The ratio of protons to electrons in all normal atoms.
Solution :
Here normal atom means neutral atoms i.e atoms whose charge is zero.
Atom charge is zero when number of proton are equal to number of electrons.
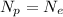
So,
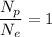
Therefore, the ratio of protons to electrons in all normal atoms is 1.
Hence, this is the required solution.