Answer:
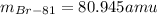
Step-by-step explanation:
Hello,
In this case, since the percent abundance of Br-79 is 51.1% and that of the Br-81 is 48.9 %, we can write:
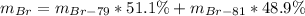
Taking into account that the mass of bromine from the periodic table is 79.909 amu, the mass of Br-81 turns out:
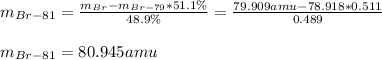
Best regards.