Answer:
n=1 holds two electrons and n=2 holds eight electrons.
Step-by-step explanation:
Hello
In this case, since the atomic number of aluminum is 13, its electron configuration is:
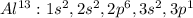
In such a way, we can see that the level n=1 is filled with two electrons since the subshell s is able to hold two electrons and the level n=2 is also filled but with eight electrons as s holds two whereas p holds 6. Moreover, n=3 is holding three electrons.
Best regards.