Answer:
F. [5]
Step-by-step explanation:
Valence electrons can be defined as the number of electrons present in the outermost shell of an atom. Valence electrons are used to determine whether an atom or group of elements found in a periodic table can bond with others. Thus, this property is typically used to determine the chemical properties of elements.
Hence, the expected number of valence electrons for an atom of a group 5A element is five (5). The elements found in group 5A typically have two (2) valence electrons in their S-orbital and three (3) valence electrons in their P-orbital, which is given by;
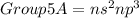
Some examples of elements found in group 5A of the periodic table are nitrogen, phosphorus, antimony etc.