Answer:
The two isotopes of Molybdenum have equal natural abundance
Step-by-step explanation:
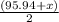 = 95.95 amu
= 95.95 amu
x = (95.95 * 2) - 95.94
= 95.96 amu is isotope B, while 95.94amu is isotope A
Using the formula x + (1 - x) = 1
95.96x + 95.94 (1-x) = 95.95
95.96x + 95.94 - 95.94x = 95.95
0.02x = 0.01
x = 0.5
∴ Isotope A and B have equal abundance since 0.5 = 50% abundance.
i hope this was helpful.