Answer:
The percentage by mass of a solution with 30.0g salt dissolved in water to make 650.0g 0f solution is 4.61 %
Step-by-step explanation:
The mass percentage of a solution is a measure of concentration that indicates the number of grams of solute that there are per 100 grams of solution.
The percent by mass is calculated as the mass of a component divided by the total mass of the mixture, multiplied by 100 to give a percentage.
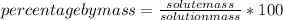
In this case:
- solute mass: 30 g
- solution mass: 650 g
Replacing in the definition of percent by mass:
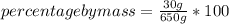
Solving:
percent by mass= 4.61 %
The percentage by mass of a solution with 30.0g salt dissolved in water to make 650.0g 0f solution is 4.61 %