Answer:
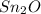
Step-by-step explanation:
Hello,
In this case, given the mass of the sample and mass of tin we can compute the mass of oxygen via:
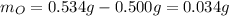
Thus, by using the atomic bas of tin and oxygen we can compute their moles:
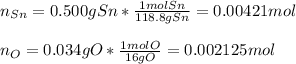
Next, we need to divide both moles by the moles of oxygen as those are the smallest in order to compute the subscript in the chemical reaction:

Therefore, empirical formula of the oxide should be:
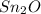
Best regards.