Answer:
V₂ = 150 mL
Step-by-step explanation:
Given that,
Initial volume of a gas,
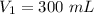
Initial pressure,
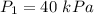
We need to find the volume of gas if the pressure is changed to 80 kPa. Boyle's law states that the relationship between pressure and volume is inverse. Mathematically,

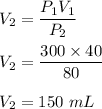
So, the new volume is 150 mL.