Answer:
1.308 x 10¹⁸ photons were emitted from the laser pointer.
Step-by-step explanation:
Given;
wavelength of the photon, λ = 5650 Å = 5650 x 10⁻¹⁰ m
power emitted by the source, P = 4 mW = 4 x 10⁻³ W
time of photon emission, t = 115 s
The energy of a single photon is given by;
E = hf
f = c / λ
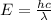
where;
c is speed of light = 3 x 10⁸ m/s
h is Planck's constant = 6.626 x 10⁻³⁴ Js
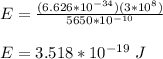
Energy of the source (laser pointer) = P x t
= (4 x 10⁻³ W) (115 s )
= 0.46 J
If no energy is lost, then emitted energy by the source must be equal to total energy of then photons;
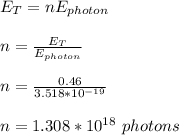
Therefore, 1.308 x 10¹⁸ photons were emitted from the laser pointer.