Answer:
Step-by-step explanation:
Hello,
In this case, for the calculation of the standard entropy of liquid lead at 500 °C (773.15 K), starting by solid lead 298.15 K we need to consider three processes:
1. Heating of solid lead at 298.15 K to 600.55 K (melting point).
2. Melting of solid lead to liquid lead.
3. Heating of liquid lead at 600.55 K (melting point) to 773.15 K.
Which can be written in terms of entropy by:
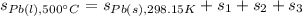
Whereas each entropy is computed as follows:
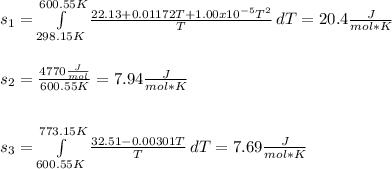
Therefore, the standard entropy of liquid lead at 500 °C turns out:
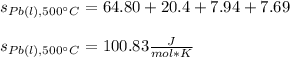
Best regards.