Answer:
0.51 %
Step-by-step explanation:
Calculated value of the amount of oxygen is 3.9 g
Theoretical value of amount of oxygen is 3.92 g
We need to find the percent error in this experiment. It is given by the difference in theoretical and Calculated value divided by theoretical and multiplying it by 100. So,
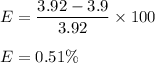
So, the percentage error is 0.51 %.