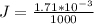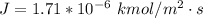Answer:
The value is
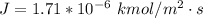
Step-by-step explanation:
From the question we are told that
The length the tube is
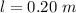
The diameter of the tube is
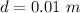
The total pressure inside the tube is
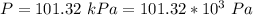
The partial pressure of CO2 at the first end is
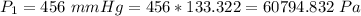
The partial pressure of CO2 at the other end is
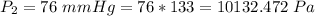
The temperature is T = 298 K
The diffusion coefficient is
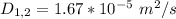
Generally the molar flux of CO2 is mathematically represented as
![J = (D_(1,2) * [P_1 -P_2])/(R* T[l])](https://img.qammunity.org/2021/formulas/chemistry/college/lhpfu9qf41kwqoptch3ucqmlox5v22pz21.png)
Here R is the gas constant with value
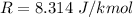
So
![J = ( 1.67 * 10^(-5) * [60794.832 -10132.472 ])/(8.314 * 298 [0.20])](https://img.qammunity.org/2021/formulas/chemistry/college/jzko66457emcvv0dvopb4pcas08uezo55p.png)
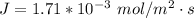
Converting to kmol