Answer:
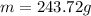
Step-by-step explanation:
Hello,
For the given chemical reaction:
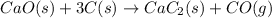
Whose heat of reaction is 464.8 kJ/mol which means that energy is absorbed due to the chemical reaction, we can compute the moles of calcium carbide via the following relationship:
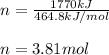
Then, since the molar mass of calcium carbide is 64 g/mol, the yielded mass turns out:
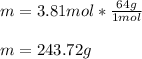
Best regards.