Answer:
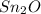
Step-by-step explanation:
Hello,
In this case, given that the mass of the product is 0.534 g, we can infer that the percent composition of tin is:
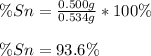
Therefore, the percent composition of oxygen is 6.4% for a 100% in total. Thus, with such percents we compute the moles of each element in the oxide:
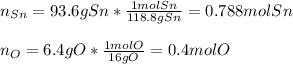
In such a way, for finding the smallest whole number we divide the moles of both tin and oxygen by the moles of oxygen as the smallest moles:
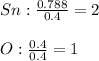
Therefore, the empirical formula is:
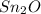
Best regards.