Answer:
3.96 × 10⁻³ mol Pb
Step-by-step explanation:
Step 1: Find molar mass
Pb (Lead) - 207.2 g/mol
Step 2: Use Dimensional Analysis
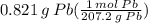 = 0.003962 mol Pb
= 0.003962 mol Pb
Step 3: Simplify
We have 3 sig figs
0.003962 mol Pb ≈ 0.00396 mol Pb
0.00396 mol Pb = 3.96 × 10⁻³ mol Pb