Answer:
0.113 M
Step-by-step explanation:
Since B and D are on opposite sides of the reaction, the concentration of D increases when the concentration of B decreases. The amount by which D increases is determined by the coefficients of B and D in the balanced chemical equation:
[D]=
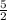 (0.045 M)=0.113 M.
(0.045 M)=0.113 M.