Given :
Actual value observed ,
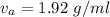 .
.
Expected value ,
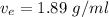 .
.
To Find :
The student's percent error.
Solution :
Percentage error is given by :
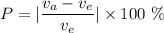
Putting given values in above equation, we get :
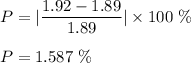
Therefore, percentage error is 1.587 %.
Hence, this is the required solution.