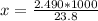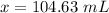Answer:
The value is
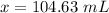
Step-by-step explanation:
From the question we are told that
The concentration of NaOH is
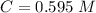
The mass of glyceryl tristearate is
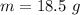
The reaction of tristearate (tristearin) with NaOH is shown on the first uploaded image (Reference Wikidot)
From the reaction we see that 1 mole of tristearate (tristearin) (with molar mass Z_b = 891.5 g/mol ) reacted with 3 moles of NaOH (with molar mass Z_a = 40 g/mol)
So the mass of tristearate (tristearin) in the equation is (m_k = 1 *891.5 = 891.5g )
and
The mass of NaOH in the equation is (m_x = 3 * 40 = 120 g )
Generally the mass of NaOH that will react with
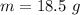 of glyceryl tristearate is mathematically represented as
of glyceryl tristearate is mathematically represented as
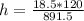
=>
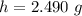
The mass of NaOH in 0.595 M ( mol/L) (i.e 0.595 mol in L(1000 mL) )of NaOH solution is mathematically evaluated as
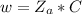
=>
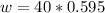
=>
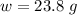
So if
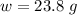 is in 1000mL of the given solution of NaOH
is in 1000mL of the given solution of NaOH
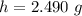 will be is x mL of the NaOH solution
will be is x mL of the NaOH solution
So