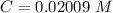Complete question
The complete question is shown on the first uploaded image
Answer:
The value is
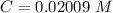
Step-by-step explanation:
From the question we are told that
The mass of NaOH is
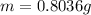
In the question DI (stands for de-ionized water )
So the number of moles of NaOH present in the given mass is
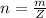
Here Z is the molar mass of NaOH with value
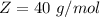
So
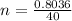
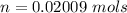
Given that the volume of the flask is V = 1 L then we can evaluated the concentration as
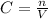
=>
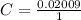
=>