Answer:
D. Nitrogen, because the ratio of moles is greater than 3:1 for H2:N2
Step-by-step explanation:
Hello,
In this case, given the reaction:
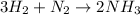
In order to identify the limiting reactant we must compute the moles of ammonia yielded by both reactants at first:
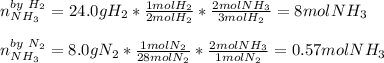
Thus, since nitrogen yields the smallest amount of ammonia as it is more heavy than hydrogen and it is in a 3:1 mole ratio for H2:N2, it is the limiting reactant, therefore the answer is D. Nitrogen, because the ratio of moles is greater than 3:1 for H2:N2.
Regards.