Answer: Lewis dot structures are models used to valence electrons.
Step-by-step explanation:
Lewis dot structures are the structures which represent the valence electrons of the element around the chemical symbol of the element.
For example : Flourine is an element which belong to group 17 and is a halogen. Atomic number of fluorine is 9 that means it contains 9 electrons, Thus the electronic configuration is represented as:
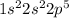
Thus the valence electrons are 7. Thus 7 electrons are represented around chemical symbol of Flourine i.e. F