Answer:
It must lose two electrons and become an ion.
Step-by-step explanation:
Hello,
In this case, for magnesium we know that the first two small balls are the first two electrons it has in the first electronic level, then, the next eight to the second level and finally the last two to the third energetic level based on its electron configuration:
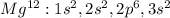
In such a way, since the magnesium atom has 12 electrons and protons, if we want to represent the Mg⁺² ion, it is clear that for it to become positive, it needs to lose two electrons so now it has 10 electrons and 12 protons, therefore, we conclude that it must lose two electrons and become an ion since it now has different number of electrons to protons.
Best regards.