Answer:

Step-by-step explanation:
Density can be found by dividing the mass by the volume.
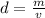
The mass is 5.28 grams and the volume is 2 cubic centimeters.
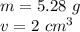
Substitute the values into the formula.
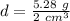
Divide.
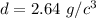
The density of the unknown substance is 2.64 grams per cubic centimeter.
The density of a diamond is about 3.5 grams per cubic centimeter. Since 2.64 is not equal to 3.5, the unknown substance is not a diamond.