Final answer:
To find the number of rhodium atoms in a 44.8 g sample, calculate the moles of rhodium by dividing the sample mass by rhodium's molar mass and then multiply it by Avogadro's number to get approximately 2.62 ×
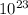 atoms.
atoms.
Step-by-step explanation:
To calculate the number of rhodium atoms in a 44.8 g sample of rhodium (Rh), you need to use Avogadro's number and the molar mass of rhodium. According to the periodic table, rhodium has an atomic mass of approximately 102.9 amu, which is its molar mass in grams per mole. Avogadro's number, which is 6.022 ×
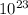 , represents the number of atoms in one mole of a substance.
, represents the number of atoms in one mole of a substance.
The number of moles of rhodium in the sample is calculated as follows:
- Divide the sample mass by the molar mass of rhodium: 44.8 g ÷ 102.9 g/mol = 0.435 moles of Rh.
- Multiply the moles of rhodium by Avogadro's number to find the number of atoms: 0.435 moles × 6.022 ×
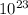 atoms/mol = 2.62 ×
atoms/mol = 2.62 ×
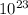 rhodium atoms (rounded to three significant figures).
rhodium atoms (rounded to three significant figures).
Therefore, a 44.8 g sample of rhodium contains approximately 2.62 ×
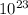 rhodium atoms.
rhodium atoms.