Answer:
M = 87.71 amu
Step-by-step explanation:
The masses of 4 isotopes of Strontium with abundance is as follows :
84 (abundance 0.50%), 86 (abundance of 9.9%), 87 (abundance of 7.0%), and 88 (abundance of 82.6%). Let M is the atomic mass of Strontium. So,
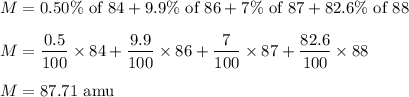
So, the atomic mass of Strontium is 87.71 amu.