Answer:
Four covalent bonds.
Step-by-step explanation:
Hello,
In this case, given the attached picture in which you can find the Lewis dot structure for metanal (formaldehyde) we can see two C-H bonds and two C-O bonds via a double bond, thus, we can compute the type of each bond given the electronegativities of hydrogen, carbon and oxygen which are 2.1, 2.5 and 3.5 respectively:
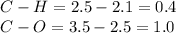
Thus, since both electronegativity difference are less 1.7 we infer that all of them are covalent, therefore, it has four covalent bonds, two C-H bonds and a double C-O bond.
Best regards-