Hello there! :)
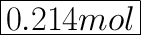
Atomic mass of KBr = 39.098 (K) + 79.904 (Br) = 119.002 grams.
Convert from grams to moles using dimensional analysis. Use the provided
amount of grams in the sample to begin:
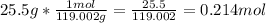
Therefore, there are approximately 0.214 moles of KBr in a 25.5 gram sample.