Answer:
The answer is
17.2 mol
Step-by-step explanation:
To find the number of moles in a substance when given the molar mass and mass we use the formula
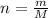
where
n is the number of moles
M is the molar mass
m is the mass of the substance
Mr( Na) = 23 , Mr( CL ) = 35.5
Molar mass ( NaCl ) = 23 + 35.5
= 58.5 g/mol
Mass = 1.005 kg
It's equivalent in grams is
1.005 × 1000 = 1005 g NaCl
So the number of moles in NaCl is
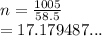
We have the final answer as
17.2 mol to the nearest tenth
Hope this helps you