Answer:
0.50 Molar or 0.50 Mol/L
Step-by-step explanation:
Molarity =
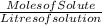
Molarity = n / V
Given: Volume of solution(V): 1.75 Litres
Moles of solute(n): 0.877
Substituting values,
Solution:
Molarity =
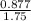
Molarity = 0.50 Molar (rounded off to the nearest decimal place)
∴ Molarity = 0.50 Molar/ 0.50 Mol/L