Answer:
Observe odor, determine pH, determine density, determine boiling point
Step-by-step explanation:
The correct procedures that would be best to use to determine whether a beaker contains only distilled water would be to observe the odor of the liquid in the beaker, determine the pH of the liquid, determine the density, and then determine the boiling point of the liquid.
Water is generally odorless and has a pH of approximately 7 with a density of 1 kg/m3 and a boiling point of 100
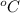 . If the liquid in the beaker ticks all these conditions, then it can be established to be only distilled water.
. If the liquid in the beaker ticks all these conditions, then it can be established to be only distilled water.