Answer:
D. 44.2 g O₂
General Formulas and Concepts:
Gas Laws
- STP (Standard Conditions for Temperature and Pressure) = 22.4 L per mole at 1 atm, 273 K
Stoichiometry
- Dimensional Analysis
- Mole Ratio
Step-by-step explanation:
Step 1: Define
Identify given.
61.9 L O₂ at STP
Step 2: Convert
We know that the oxygen gas is at STP. Therefore, we can set up and solve for how many moles of O₂ is present:
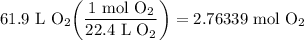
Recall the Periodic Table (Refer to attachments). Oxygen's atomic mass is roughly 16.00 grams per mole (g/mol). We can use a mole ratio to convert from moles to grams:
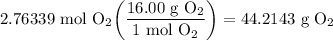
Now we deal with sig figs. From the original problem, we are given 3 significant figures. Round your answer to the exact same number of sig figs:
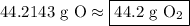
∴ our answer is letter choice D.
---
Topic: AP Chemistry
Unit: Stoichiometry