Answer:
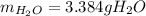
Step-by-step explanation:
Hello,
In this case, the chemical reaction is:
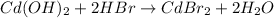
Thus, we first identify the limiting reactant by computing the yielded moles of water by both of the reactants:
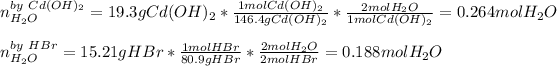
In such a way, since HBr yields less water than cadmium hydroxide, we infer that HBr is the limiting one, therefore, the yielded mass of water are:
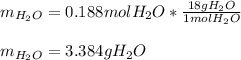
Regards.