Answer:
Pyrite.
Step-by-step explanation:
In this case, for the given samples, we compute the densities considering that the volume of each sample is computed by the difference between the volume of the water + sample and the volume of water:
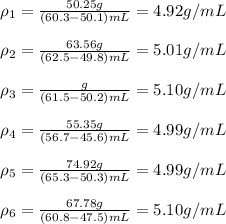
Therefore, since the density of the samples are closer to the density of pyrite (5.01 g/cm³) we conclude that the samples are more like to be pyrite.
Best regards.