Solution:
The data provided in the question are :
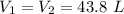
=
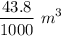
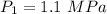 and
and
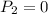
Initial pressure of neon = 1.1 MPa
Final Pressure = 346 kPa
Initial temperature of neon = 298 K

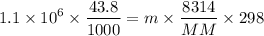
Molecular mass of neon = 20.1797 g/mole
m = 0.3924 kg
For final temperature:
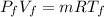
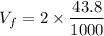
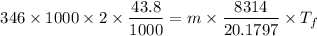
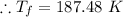
a). From first law of thermodynamics :
δQ = δU + δW
Tds = dU + PdV
or dH = dTs + VdP
As system is insulator, Tds = 0
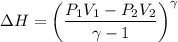 as
as
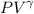 = constant
= constant
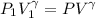
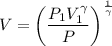
Substituting in VdP and integrating, the above equation is obtained.
So, γ = 1.67 (mono atomic neon)
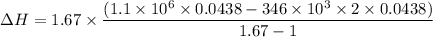
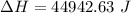
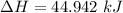
b). Easier way is :
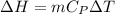
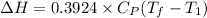
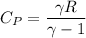
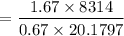
= 1026.92 J/kg-K
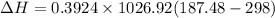
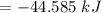
The negative sign indicates decrease in enthalpy.
The answer by easier way is very near to the value in part (a).
Error (%) =
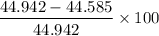
= 0.015 % (which is negligible)
Therefore, both the answers are same.