Answer:
The new pressure is 4 times of the initial pressure.
Step-by-step explanation:
Given that,
The pressure of an ideal gas is 4 atm.
We need to find the change in the pressure of the gas if the temperature by a factor of 4.
We know that,
PV = nKT ....(1)
P = pressure, V = volume, T = temperature
Let P' is the new pressure of the gas. So,
P'V= nK(4T) ......(2)
Dividing equation (1) and (2) we get :
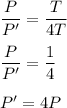
It means that the new pressure is 4 times of the initial pressure.