Answer:
The classification of the concern is listed in the interpretation segment below.
Step-by-step explanation:
(a)...
Simple cubic lattice
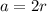
Now,
The unit cell volume will be:
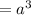
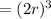
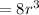
At one atom per cell, atom volume will be:
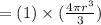
Then the ratio will be:
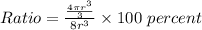
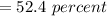
(b)...
Diamond lattice
The body diagonal will be:

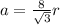
The unit cell volume will be:
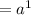
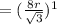
At eight atom per cell, the atom volume will be:
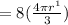
Then the Ratio will be:
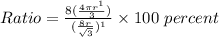
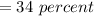
Note: percent = %