Given :
A mixture of water and acetone at 756 mm boils at 70.0°C.
The vapor pressure of acetone is 1.54 atm at 70.0°C, while the vapor pressure of water is 0.312 atm at the same temperature.
To Find :
The percentage composition of the mixture.
Solution :
By Raoult's law :
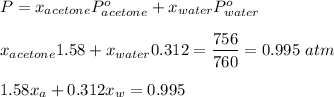 ......( 1 )
......( 1 )
Also ,
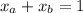 ......( 2 )
......( 2 )
Solving equation 1 and 2 , we get :
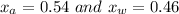 .
.
Mass of acetone ,
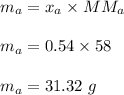
Mass of water ,
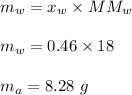
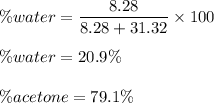
Hence , this is the required solution.