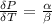Answer:
The coefficient of thermal expansion α is
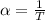
The coefficient of compressibility
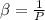
Now considering
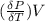
From equation (1) we have that
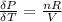
From ideal equation
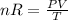
So
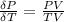
=>
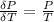
=>
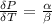
Step-by-step explanation:
From the question we are told that
The coefficient of thermal expansion is
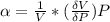
The coefficient of compressibility is
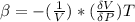
Generally the ideal gas is mathematically represented as
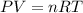
=>
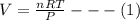
differentiating both side with respect to T at constant P
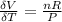
substituting the equation above into
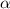
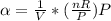
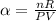
Recall from ideal gas equation
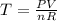
So
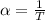
Now differentiate equation (1) above with respect to P at constant T
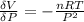
substituting the above equation into equation of
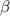
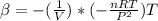
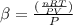
Recall from ideal gas equation that
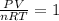
So
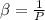
Now considering
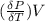
From equation (1) we have that
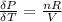
From ideal equation
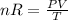
So
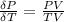
=>
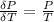
=>