Answer:
The number of moles of O atom in
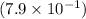 mol of
mol of
 = 1.6
= 1.6
Step-by-step explanation:
1 molecule of
 contains 2 atoms of O
contains 2 atoms of O
So,
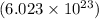 molecules of
molecules of
 contains
contains
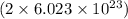 atoms of O.
atoms of O.
We know that 1 mol of an atom/molecule/ion represents
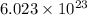 numbers of atoms/molecules/ions respectively.
numbers of atoms/molecules/ions respectively.
So,
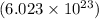 molecules of
molecules of
 is equal to 1 mol of
is equal to 1 mol of
 .
.
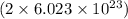 atoms of O is equal to 2 moles of O atom.
atoms of O is equal to 2 moles of O atom.
Hence, 1 mol of
 contains 2 moles of O atom.
contains 2 moles of O atom.
Therefore,
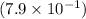 mol of
mol of
 contains
contains
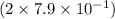 moles of O atom or 1.6 moles of O atom.
moles of O atom or 1.6 moles of O atom.