Answer:
* Limiting reactant: K2PtCl4.
*
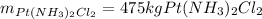
Step-by-step explanation:
Hello,
In this case, for the reaction:
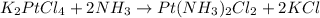
By starting with 655.1 kg of K2PtCl4 (molar mass 415.1 g/mol) and 728 kg of NH3 (molar mass 17 g/mol) the limiting reactant is identified as the one yielding the smallest moles of cisplatin, thus, we proceed as follows:
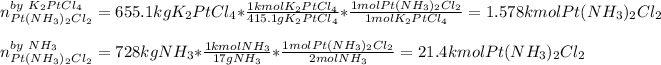
In such a way, we infer that the K2PtCl4 is the limiting reactant, therefore, the maximum amount of cis-plat (300 g/mol) produced is:
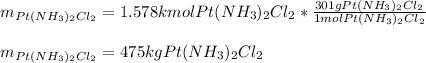
Best regards.