Answer:
The redox reaction in the question is missing. The reaction is :
Cl 2(g) + Mn 2 +(aq) + 2 H-On â 2 Cl-(aq) + MnO2(s) + 4 H + (aq)
2.59V
Explanation:
A redox reaction is also known as oxidation - reduction reaction. In redox reaction, there is a transfer of electrons between the species. In one species there is oxidation and in the other species there is reduction process.
Cathode half reaction equation:
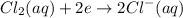
Anode half reaction equation:
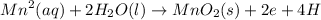
E°cathode= 1.36V
E°anode= -1.23V
E°cell= E°cathode - E°anode
E°cell= 1.36 - (-1.23)
E°cell= 2.59V