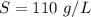Answer:
The answer is YES
The value is
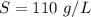
Step-by-step explanation:
From the question we are told that
The volume of the sample taken is v = 13.0 mL
The temperature is
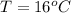
The mass of the sample is
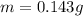
Generally the solubility of the substance X is mathematically represented as
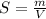
=>
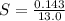
=>
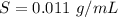
=>