Answer:
Diffusive force J = 9590
Step-by-step explanation:
From the given information:
Concentration gradient C₁ - C₂ = 35 mM = 35 × 10⁻³ M
membrane width Δ x = 1 cm = 10⁻² m
Solubility S = 1
diffusive area A = 10
Temperature T = 1° C = (273 + 1 )K = 274 K
Using the expression:
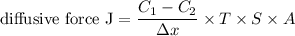
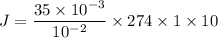
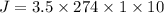
Diffusive force J = 9590