Given :
On a shelf sits a bottle of NaCl solutions with a molar concentration of 2.50 M and a total volume of 300 mL. If 15.0 mL of the solution is added to 985 mL of pure water .
To Find :
The molar concentration of NaCl in the new solution.
Solution :
Molarity M is given by :
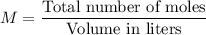
Now , 1000 ml of NaCl contains 2.5 moles of NaCl .
So , 15 ml of NaCl contains :
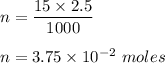
New , volume V = 15 + 985 = 1000 ml = 1 L .
So , putting value of n and V in above equation :
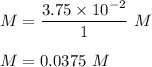
Therefore , the molar concentration of NaCl in the new solution is 0.0375 M .
Hence , this is the required solution .