Balanced chemical reaction is :

Also 30.2 g of CaO is added to 34.5 g of HCl and 6.35 g of water is formed.
Now , percentage yield is given by :
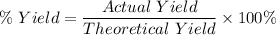
Moles of CaO ,
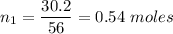
Moles of HCl ,
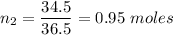
Now , 2 moles of HCl react with 1 mole of
 .
.
So , moles of
 :
:
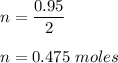
Mass of water produced :
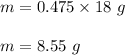
But in practical 6.53 g of water is produced .
So ,
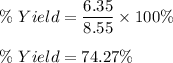
Therefore, percent yield is 74.27 %.
Hence, this is the required solution.