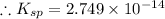Answer: 2.749 x 10^-14
Step-by-step explanation:
Given:
The concentration of Ce(IO3)4 in the solution = 1.5 x 10-2 g/100 mL= 0.15 g/1000 mL= 0.15 g/L
Molar mass of Ce(IO3)4 = 839.7267 g/mol
Therefore, the molarity of Ce(IO3)4 in the solution is given by:
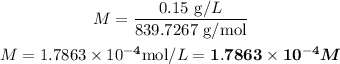
The solubility equilibrium is given by:
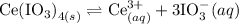
Therefore, the solubility product is given by:
![\begin{gathered}K_(s p)=\left[C e^(3+)\right] \cdot\left[I O_(3)^(-)\right]^(3) \\\therefore K_(s p)=\left(1.7863 * 10^(-4)\right) *\left(3 * 1.7863 * 10^(-4)\right)^(3)\end{gathered}](https://img.qammunity.org/2023/formulas/chemistry/college/hp0py0570ftll7k6ck4ohwp7cku8djpwl2.png)