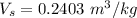Answer:
The weight in (pounds ) is
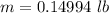
The specific volume is
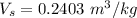
Explanation:
From the question we are told that
The temperature is
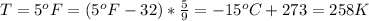
The volume is
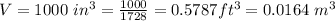
The initial absolute pressure is
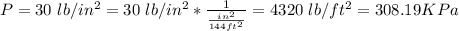
Generally from ideal gas equation we have
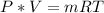
Here m is the weight nose wheel tire in pounds
R is the gas constant of air with value
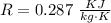
So
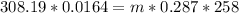
=>
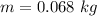
Converting to pounds
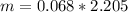
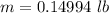
Form this equation
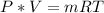 specific volume is
specific volume is
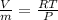
=>
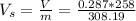
=>